GhostChemist
Expert
- Language
- 🇬🇧
- Joined
- Nov 20, 2022
- Messages
- 114
- Reaction score
- 246
- Points
- 43
The synthesis of Ephedrine hydrochloride is proceeding according to Scheme 1.
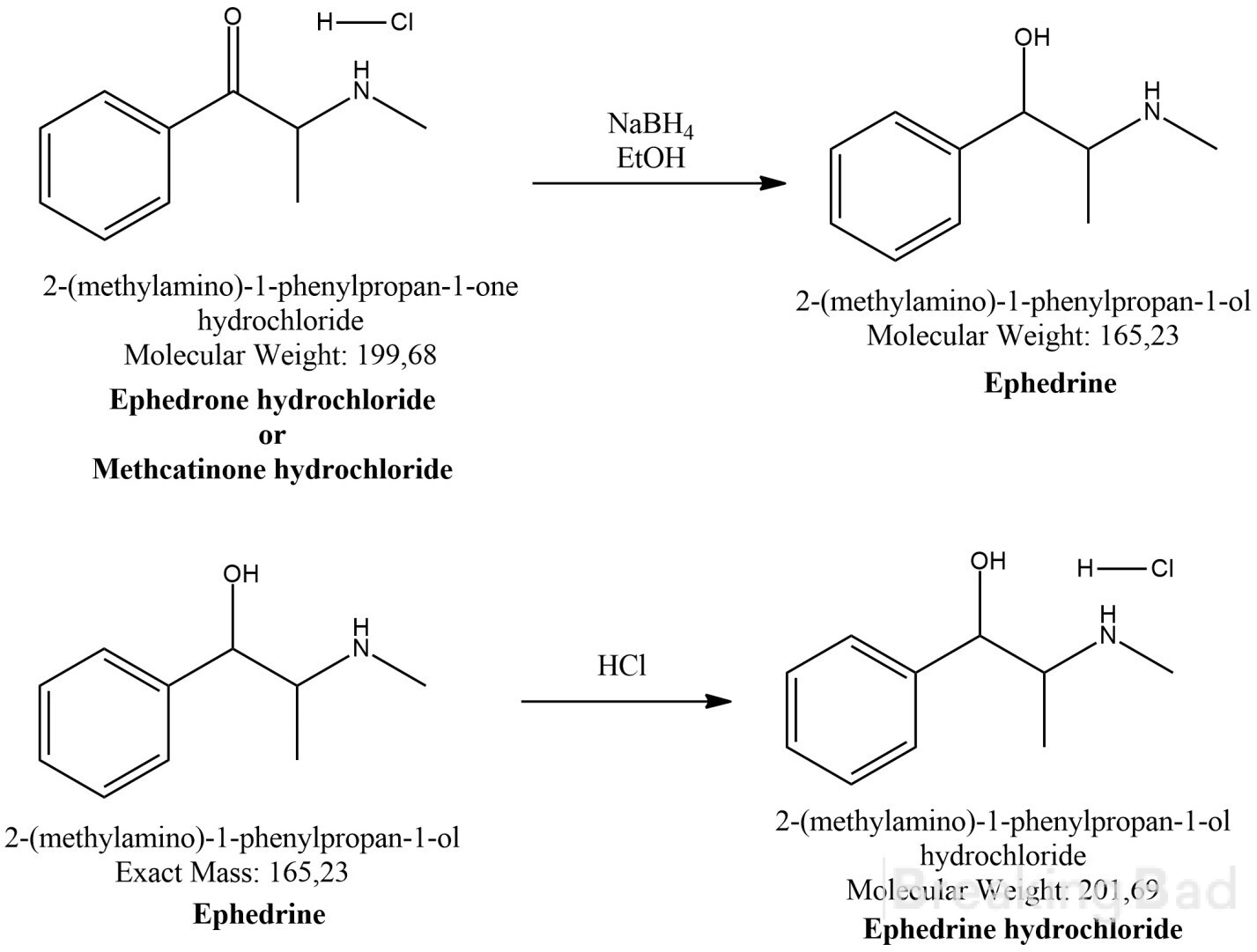
Scheme 1
Scheme 1
Were've carried out 3 synthesis of Ephedrine with 1, 1.5 and 2 equivalents of NaBH4 to methcathinone
Starting reagents and materials for a one synthesis:
- 10 g of purified methcathinone hydrochloride
- 40-50 mL of 88% ethanol
- 2 g (1 equivalent (Eq)), 2.95 g (1.5 Eq) and 3.7 g (2 Eq) of sodium borohydride
- 300 mL of distilled water
- 250-300 + 20 mL of Dichloromethane (DCM)
- 15 mL of 14% hydrochloric acid
- 10-15 mL of ethyl acetate
- 10 mL of cold acetone (temperature not exceeding -5°C)
- Magnetic stirrer with heating
- 1 L three-necked flask
- Beakers
- Separatory funnel
- Reflux Condenser
- Thermometer
- Glass corks
- pH indicator paper.
- 31
Ephedrine Hydrochloride Synthesis Via NaBH4 From Ephedrone Hydrochloride...
The starting components are presented in Figure 1.
Fig 1
Methcathinone hydrochloride is placed into the flask, and ethanol is added until dissolved. Figure 2
Fig 2
After complete dissolved of methcathinone, sodium borohydride is added in very small portions. The reaction mixture temperature should be in the range of 20-30°C. Figure 3
Fig 3
After the addition of the entire amount of sodium borohydride, the reaction mixture is stirred for 24 hours. Figure 4
Fig 4
After 24 hours, 200 mL of water is added to the reaction mixture until most of the precipitate dissolves. Figure 5
Fig 5
After the addition of water, the mixture is stirred for approximately 20-30 minutes. Figure 6
Fig 6
To the obtained solution, 50 mL of DCM were added. Figure 7
Fig 7
The mixture with added DCM was stirred for 5-10 minutes. Figure 8
Fig 8
The obtained mixture is transferred in portions of 50-100 mL into a separatory funnel and extracted with additional portions of DCM. The total volume of DCM for extraction is 250-300 mL. Figure 9
Fig 9
The obtained ephedrine extract in DCM is transferred to a flask, and 50 mL of water is added. The extract in DCM should have an alkaline pH. Figure 10
Fig 10
To the reaction mixture, 14% hydrochloric acid (12-17 mL in experiment 1.5 eq and for Met-reduction) is added dropwise until a consistently slightly acidic pH was obtained. Figure 11
Fig 11
After the addition of the hydrochloric acid solution and reaching a stable slightly acidic pH, the reaction mixture was stirred for 30-40 minutes. Figure 12
Fig 12
After stirring the mixture for 30-40 minutes, the mixture is allowed to separate. Figure 13
Fig 13
The layer of DCM was separated and again added 50 mL of water to extract the ephedrine hydrochloride. If the solution has an alkaline or neutral pH, a small portion of hydrochloric acid is added. The pH should be slightly acidic. Figure 14
Fig 14
The aqueous layers with an acidic pH are combined, and 20 mL of DCM was added to them for purification from organic impurities. Figure 15
Fig 15
The mixture is shaken and left for layer separation in a separatory funnel. Figure 16
Fig 16
The layer of DCM was discarded. The aqueous layer was filtered through a paper filter. Figure 17
Fig 17
The filtered solution was evaporated at a temperature of 100-130°C. Figure 18
Fig 18
Evaporation continues until crystallization occurs. Figure 19
Fig 19
To confirm the identity of the Ephedrine, a qualitative test was conducted. A few ml of CuSO4 solution, a few drops of NaOH solution, and 2-3 mL of ethyl acetate are added to the aqueous solution of the crystal. Ephedrine produces a pink coloration in the ethereal layer (Test Tube 2). In comparison, methcathinone does not show such a reaction (Test Tube 1). Additionally, when the obtained product is heated with a solution of K3[Fe(CN)6] and alkali, it produces the smell of benzaldehyde, that is indicating the presence of ephedrine. Ephedrine hydrochloride crystals have a bitter taste. Figure 20
Fig 20
The crystals are transferred to a filter and treated with a mixture of 15 mL of ethyl acetate and 10 mL of cold acetone (not exceeding -5°C). The mixture is quickly and thoroughly stirred. The crystals are allowed to settle for 20-30 seconds and then vacuum-filtered. The washed crystals are air-dried. Figure 21
Fig 21
The yield of ephedrine hydrochloride is 6.1-7.6 g or 60 - 75%
Analysis
Reagents and materials
- Ephedrine hydrochloride synthesis by 1.5 Eq; 2 Eq and 1 Eq NaBH4
- Simon`s reagent (2% water solution of Na2CO3; 1% water solution of sodium nitroprusside Na2[Fe(CN)5NO]*2H2O; solution of acetaldehyde in ethanol)
- Reagent for Chen-Kao reaction (1% water solution of acetic acid; 1% water solution of CuSO4*5H2O; 2N water solution of NaOH)
- Reagent Ninhydrin (10% solution of Ninhydrin in ethanol)
- NaOH
- Ethyl acetate
- Ethanol 88%
- TLC Silica gel
- UV-lamp
- Glass Pasteur pipettes
- Refractometer
- Watch glasses
- Measuring flask 100 ml 20 ℃ (B)
- Distilled water
3 samples of Ephedrine hydrochloride synthesis by 1.5 Eq; 2 Eq and 1 Eq NaBH4 were obtained. Fig 22
Fig 22
All obtained samples of Ephedrine hydrochloride were tested with the qualitative Simon's reaction. All ephedrine samples showed a negative reaction for the presence of meth. As a comparison, a control determination was carried out. Metcat also did not exhibit a qualitative Simon's reaction; it is possible that it is not specific to katinons. Fig 23
Fig 23
Subsequently, the Chen's reaction was conducted. All samples of Ephedrine hydrochloride and sample of Methcathinone exhibited a positive reactions. Fig 24
Fig 24
The obtained samples of Ephedrine hydrochloride were tested using thin-layer chromatography with visualization under a UV lamp. Prepared aqueous solutions of Ephedrine hydrochloride were applied to the plate and dried. Fig 25
Fig 25
Subsequently, the plate was placed in a mixture of solvents (50 mL of 88% ethanol, 25 mL of water, 5 mL of ethyl acetate, and 20 mL of 0.5% NaOH solution). Fig 26
Fig 26
Under UV irradiation, only marks of the main substance are visible. Fig 27
Fig 27
The second chromatogram was obtained in a solvent mixture of 70 mL of 88% ethanol and 10 mL of ethyl acetate. Fig 28
Fig 28
Visualization under the UV lamp revealed the presence of a single component and a very small amount of impurities. Fig 29
Fig 29
Next, the plate was sprayed with a ninhydrin solution. Fig 30
Fig 30
The plate was dried, and visualization was carried out by heating the plate to 120°C. Fig 31
Fig 31
Considering the very close chemical relationship between Methcathinone and Ephedrine, achieving a clear separation on the chromatogram is quite challenging. The most reliable method is measuring the refractive index of aqueous solutions of Ephedrine prepared with precisely defined concentrations. For this purpose, solutions were prepared with concentrations: 1st sample - 1.5 Eq 1% (1 g in 100 mL), 2nd - 2 Eq 5% (5 g in 100 mL), 3rd - 1 Eq 8% (8 g in 100 mL). Fig 32
Fig 32
Refractive index values for solutions of Ephedrine hydrochloride with precisely defined concentrations are provided in Table 1.
Table 1. Concentrations of Ephedrine hydrochloride solutions and refractive index
Refractive index nD20 | Concentrations of Ephedrine hydrochloride, % |
1.334 | 0.5 |
1.335 | 1 |
1.336 | 1.5 |
1.337 | 2 |
1.338 | 2.5 |
1.339 | 3 |
1.340 | 3.5 |
1.341 | 4 |
1.342 | 4.5 |
1.343 | 5 |
1.344 | 5.5 |
1.345 | 6 |
1.346 | 6.5 |
1.347 | 7.5 |
1.348 | 8 |
1.349 | 8.5 |
1.350 | 9 |
1.351 | 9.5 |
1.352 | 10 |
1.353 | 10.5 |
For the 1.5 Eq solution with a concentration of 1%, the refractive index was 1.3349. Fig 33
Fig 33
For the 2 Eq solution with a concentration of 5%, the refractive index was 1.3429. Fig 34
Fig 34
For the 1 Eq solution with a concentration of 8%, the refractive index was 1.3493. Fig 35
Fig 35
Based on the obtained data, the use of 1 equivalent of sodium borohydride leads to the presence of impurities in the sample after synthesis. Subsequently, it is necessary to use no less than 1.5 equivalents of borohydride for the complete reduction of Methcathinone to Ephedrine. The Raman spectroscopy spectrum of the racemic Ephedrine hydrochloride sample is presented in Fig 36.
Fig 36
Last edited by a moderator:
