- Language
- 🇺🇸
- Joined
- May 10, 2025
- Messages
- 168
- Reaction score
- 22
- Points
- 18
Product Name MYRISTICIN ALDEHYDE

MYRISTICIN ALDEHYDE Price
Product number
PackagingPrice
Product description
Buy
Alfa Aesar
L08213
1g$47.65
5-Methoxypiperonal, 97%
Buy
Alfa Aesar
L08213
5g$179
5-Methoxypiperonal, 97%
Buy
Alfa Aesar
L08213
25g$590.65
5-Methoxypiperonal, 97%
Buy
TRC
M260955
10g$745
7-Methoxy-1,3-benzodioxole-5-carboxaldehyde
Buy
American Custom Chemicals Corporation
CHM0030883
500MG$796.95
MYRISTICIN ALDEHYDE 95.00%
Buy
**There are several Suppliers, mainly from China **
 m.chemicalbook.com
m.chemicalbook.com
THIS IS SUCH A FASCINATING VERY VERY VERY VERY VERY HIGH POTENTIAL SEXY MOLECULE
THE BEST DEALS IN TOWN ARE GOING TO BE 5 G FOR $100 ...
MYRISTICIN ALDEHYDE

Myristicin
(3-methoxy-4,5-methylenedioxyallylbenzene)
5-methoxy-3,4-methylenedioxy-allylbenzene

Isomyristicin
1-Methoxy-2,3-methylenedioxy-5-(1-propenyl)benzene
(E)-1-Methoxy-5-propenyl-2,3-(methylenedioxy)benzene
The isomer of myristicin

Benzaldehyde


Piperonal
3,4-Methylenedioxybenzaldehyde
1,3-Benzodioxole-5-carbaldehyde
Piperonyl aldehyde

MYRISTICIN ALDEHYDE
5-Methoxypiperonal
3-Methoxy-4,5-(methylenedioxy)benzaldehyde)
7-methoxy-1,3-benzodioxole-5-carbaldehyde

CAS Number: 5780-07-4
Chemical Formula: C9H8O4
Molecular Weight: 180.16 g/mol
Melting point: 126-132°
Sensitivity: Air Sensitive
Solubility: Insoluble in water.
1 gram $60

MYRISTICIN ALDEHYDE
...
Synthesis of Myristicinaldehyde
"... But from nutmeg oil ?!?! YOU GOT TO BE CRAZY !!! LOL ! ... "
... Suppose SWIM was an eager Beeeee.
This Beeee would UTFSE & experiment with an Essential Oil Distillation of Nutmeg oil (or Mace oil) .
This Beeee read of this weblink https://www.erowid.org/archive/rhodium/chemistry/nutmeg.myristicin.html
"The aromatic ether fraction of oil of nutmeg has been previously shown1 to consist of eugenol (Ia), isoeugenol (IIa), safrole (Ic) and myristicin (Id). Vacuum distillation yields a fraction (bp 109-112°C/1 mmHg; 60g from 1 kg of "W.I." oil of nutmeg (George Lueders and Co.)) which consisted of a substance heretofore accepted both chemically1,2 and pharmacologically3 as the single compound, myristicin (Id)."
According to Alexander S., " (from Oil of Nutmeg) The careful distillation of Oil of Nutmeg (or the Oil of Mace) allowed the isolation of a number of compounds in varying degrees of purity. The fraction that boiled in the 110-115 °C range at about 1.0 mm/Hg was myristicin (3-methoxy-4,5-methylenedioxyallylbenzene). It constituted some 7% of the original oil of commerce and, in its original isolated form, was obtained with a purity of 87%. The major contaminant was elemicin (3,4,5-trimethoxyallylbenzene). "
This Beeee has a theory ~7-12% of a bottle of Nutmeg oil distilled will give the precious MYRISTICIN .
.
This Beeeee continued to read "The isomerization of this fraction with alcoholic potassium hydroxide yielded (trans) isomyristicin"
This Beeeee read from another weblink
https://www.erowid.org/archive/rhodium/chemistry/myristicinaldehyde.html
and another weblink https://www.erowid.org/library/books_online/pihkal/pihkal132.shtml
"The syntheses have actually started from myristicinaldehyde (3-methoxy- 4,5-methylenedioxybenzaldehyde) (IV) produced by isomerisation of myristicin to isomyristicin and subsequent oxidation"
This Beeee couldn't find a direct scientific layout of [Isomyristicin ---> Myristicinaldehyde]
...soooo... :/ ... however, SWIM came across a weblink "isosafrole to piperonal" that may be relative ... https://www.erowid.org/archive/rhodium/chemistry/piperonal.isosafrole.html
This Beeeeeee read from this link "
[ Back to the Chemistry Archive ]
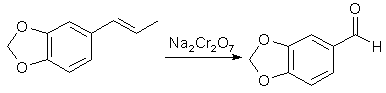
Since the initial materials are not completely miscible with water, the effect of introducing a dispersing agent was studied; for this purpose sulphanilic acid (which had the additional advantage of protecting the aldehyde when formed from further oxidation) and also Dispersol were used. The oxidation of isosafrole to piperonal by potassium permanganate under tested reaction conditions was far too vigorous, and the main product was piperonylic acid, whereas sodium dichromate and sulphuric acid afforded a 70% yield of piperonal, which was increased to 80% and 86.5% by the use of Dispersol and sulphanilic acid respectively as dispersing agents. The higher yield promoted by the sulphanilic acid was ascribed to ephemeral formation of a Schiff's base with the aldehyde when formed. With isoeugenol, in which the benzene structure is not immobilized by the clamping arrangement of the methylenedioxy group, the chromic acid oxidation gives a yield of 67% of vanillin as compared with the 86.5% of piperonal obtained from isosafrole.
A mixture of isosafrole (32.4g, 0.2 mol), 50% aqueous sulphuric acid (160 g) and water (1 L) at 30-40°C was vigorously stirred during the gradual addition over 30 minutes of a solution of sodium dichromate (44g, 0.13 mol, +10% excess) in water (200 ml). Reduction of the dichromate to green chromium salt appeared to be almost immediate; the mixture was twice extracted with benzene (600 ml), and the combined extracts were washed with 5% aqueous sodium hydroxide (200ml) followed by water (500ml). The extract was then dried over anhydrous calcium chloride, and the benzene removed, when the light brown residual oil crystallised on keeping (26g). The crude product was refluxed with ethyl alcohol (200 ml) and animal charcoal (10g), the crude solution filtered hot, and the bulk reduced to 100ml, when the piperonal was obtained in white crystals, mp 36-37°C (yield 21 g of pure product, 70%).
(b) With sulphanilic acid as dispersing agent.
Details were as in (a), except that the isosafrole was added to a solution of sulphanilic acid (12g) and sulphuric acid (80g, d 1.84) in water (1 L). Yield of pure product, 26 g. (86.5%). (c) Details were as in (a) except that Dispersol (5g) was present in the oxidation medium. Yield of pure product 24g (80%).
Experimental--Variations in temperature and in amount of oxidizing agent used:
"
This Beeeee is sure Isomyristicin ----> Myristicinaldehyde
...
This Beeeee believes this weblink https://www.erowid.org/archive/rhodium/chemistry/myristicinaldehyde.shulgin.html
may be off topic
but [Isomyristicin ------> 1-(3-Methoxy-4,5-methylenedioxyphenyl)-2-nitropropene]
via tetranitromethane
This Beeeee continued reading this discourse " Experimental
1-(3-Methoxy-4,5-methylenedioxyphenyl)-2-nitropropene (2)
A solution of 50 g isomyristicin (1) in 300 ml dry acetone and 24 g pyridine was cooled to 0°C with vigorous stirring. There was then added 54 g of cold tetranitromethane which caused a slight temperature rise (to 5°C) despite the external cooling. The stirring was continued for 2 min at which time the reaction mixture was quenched with a chilled solution of 16.8 g KOH in 300 ml H2O. Stirring was continued while the heat of neutralization dissipated. and the product was then removed by filtration. An additional quantity of the nitropropene (for a total of 50.7 g, 82%) was obtained from the filtrates by extraction with methylene chloride. The low melting point (103°C) was due to a small contamination with myristicinaldehyde which is generated concurrently. Although it cannot interfere with the subsequent reaction below, it can be removed by recrystallization from methanol to yield yellow needles of 2, mp 110°C."
This Beeeee along with the nitropropene discourse, may be simularly conducted with Apiolealdehyde
"
Apiolealdehyde (4, R = OCH3)
Employing the procedure described above for isomyristicin, isoapiole (mp 55–56°C obtained from apiole of Oil of Parsley) was nitrated to 1-(2,5-dimethoxy-3,4 methylenedioxyphenyl)-2-nitropropene, and this intermediate was similarly converted to the aldehyde. This product aldehyde (mp 102–103°C) was obtained in a 75% overall yield from isoapiole."
This Beeeee had ended the off subject discourse and we continue with the
The MYRISTICIN ALDEHYDE.
This Beeeee came into conclusion of this 'aromatic aldehyde' is more rare than of its chemical brother Heliotropin, just guessing there wasn't any aromatherapy fragrance public usage ???
Too much extractions and scientific methods for public corporations to care to spend researching upon this very delicate rare "nutmeg aldehyde"???
Legitimate reason and usage for these special aldehydes ???
Any actual real photo pictures or videos of this aromatic aldehyde ???
What reason of the expensive price ???
What reason of so little scientific notes ???
*********?????????...
MAY SOMEBODY OUT THERE ADD SOME REFERENCES OR SCIENTIFIC LITERATURE
OF
PROPENYLBENZENE OXIDATION VIA potassium permanganate and sodium/potassium dichromate TO ALDEHYDE
...???????????************
??????????? Aromatic allylbenzene ------>
Aromatic aldehyde ????????
Soooo little is known!
This Beeeee believes conducting the propenylbenzene oxidation to the aromatic aldehyde would have more product and save lesssssss $ than to buy those few grams for atleast hundreds of dollars.
Yet Myristicinaldehyde, Piperonal, Apiolealdehyde,
2,4,5-trimethoxy-benzaldehyde (asarone-like),
3,4,5-trimethoxybenzaldehyde (elemicin -like) and more aldehydes have quite a curious fascination, potential for organic chemistry, and are indeed worth a look.
This Beeeee would like to thank the ladies and gentlemen for taking time to read about this interesting science phenomenal.
Thank you
REFERENCES for 3-Methoxy-4,5-methylenedioxybenzaldehyde (Myristicinaldehyde)
Product Link
Myristicin Aldehyde MSDS
5-Methoxypiperonal MSDS
"Nutmeg Benzaldehyde" Chemical & Synthesis Info
 www.chemsrc.com
www.chemsrc.com
A Total Synthesis of Myristicinaldehyde
A Total Synthesis of Myristicin
Benzaldehydes from Propenylbenzenes
Reference: Davies and Hodgson, J.S.C.I., June 1943, 91-93.
Alexander & Ann Shulgin
P.I.H.K.A.L.
#132 MMDA
3-METHOXY-4,5-METHYLENEDIOXYAMPHETAMINE
Alexander & Ann Shulgin
P.I.H.K.A.L.
#109 MDMA
MDM; ADAM; ECSTASY; 3,4-METHYLENEDIOXY-N-METHYLAMPHETAMINE

Chemical Properties
| Melting point | 130-132°C |
| Boiling point | 110-115 °C(Press: 0.5 Torr) |
| Density | 1.321±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| form | solid |
| Water Solubility | Insoluble in water. |
| Sensitive | Air Sensitive |
| BRN | 384077 |
| LogP | 1.310 (est) |
| CAS DataBase Reference | 5780-07-4(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3-Benzodioxole-5-carboxaldehyde, 7-methoxy- (5780-07-4) |
| UNSPSC Code | 12352212 |
MYRISTICIN ALDEHYDE Price
Product number
PackagingPrice
Product description
Buy
Alfa Aesar
L08213
1g$47.65
5-Methoxypiperonal, 97%
Buy
Alfa Aesar
L08213
5g$179
5-Methoxypiperonal, 97%
Buy
Alfa Aesar
L08213
25g$590.65
5-Methoxypiperonal, 97%
Buy
TRC
M260955
10g$745
7-Methoxy-1,3-benzodioxole-5-carboxaldehyde
Buy
American Custom Chemicals Corporation
CHM0030883
500MG$796.95
MYRISTICIN ALDEHYDE 95.00%
Buy
**There are several Suppliers, mainly from China **

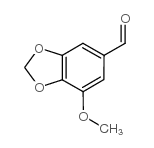
MYRISTICIN ALDEHYDE | 5780-07-4
MYRISTICIN ALDEHYDE (CAS 5780-07-4) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight, uses, prices, suppliers, SDS and more, available at Chemicalbook.
THIS IS SUCH A FASCINATING VERY VERY VERY VERY VERY HIGH POTENTIAL SEXY MOLECULE
THE BEST DEALS IN TOWN ARE GOING TO BE 5 G FOR $100 ...
MYRISTICIN ALDEHYDE
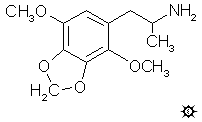
Myristicin
(3-methoxy-4,5-methylenedioxyallylbenzene)
5-methoxy-3,4-methylenedioxy-allylbenzene
Isomyristicin
1-Methoxy-2,3-methylenedioxy-5-(1-propenyl)benzene
(E)-1-Methoxy-5-propenyl-2,3-(methylenedioxy)benzene
The isomer of myristicin
Benzaldehyde
Piperonal
3,4-Methylenedioxybenzaldehyde
1,3-Benzodioxole-5-carbaldehyde
Piperonyl aldehyde
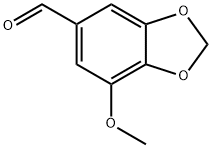
MYRISTICIN ALDEHYDE
5-Methoxypiperonal
3-Methoxy-4,5-(methylenedioxy)benzaldehyde)
7-methoxy-1,3-benzodioxole-5-carbaldehyde
CAS Number: 5780-07-4
Chemical Formula: C9H8O4
Molecular Weight: 180.16 g/mol
Melting point: 126-132°
Sensitivity: Air Sensitive
Solubility: Insoluble in water.
Notes
Air Sensitive. Incompatible with air, oxidizing agents. Store under dry inert gas. Store away from air.1 gram $60
MYRISTICIN ALDEHYDE
...
Synthesis of Myristicinaldehyde
"... But from nutmeg oil ?!?! YOU GOT TO BE CRAZY !!! LOL ! ... "
... Suppose SWIM was an eager Beeeee.
This Beeee would UTFSE & experiment with an Essential Oil Distillation of Nutmeg oil (or Mace oil) .
This Beeee read of this weblink https://www.erowid.org/archive/rhodium/chemistry/nutmeg.myristicin.html
"The aromatic ether fraction of oil of nutmeg has been previously shown1 to consist of eugenol (Ia), isoeugenol (IIa), safrole (Ic) and myristicin (Id). Vacuum distillation yields a fraction (bp 109-112°C/1 mmHg; 60g from 1 kg of "W.I." oil of nutmeg (George Lueders and Co.)) which consisted of a substance heretofore accepted both chemically1,2 and pharmacologically3 as the single compound, myristicin (Id)."
According to Alexander S., " (from Oil of Nutmeg) The careful distillation of Oil of Nutmeg (or the Oil of Mace) allowed the isolation of a number of compounds in varying degrees of purity. The fraction that boiled in the 110-115 °C range at about 1.0 mm/Hg was myristicin (3-methoxy-4,5-methylenedioxyallylbenzene). It constituted some 7% of the original oil of commerce and, in its original isolated form, was obtained with a purity of 87%. The major contaminant was elemicin (3,4,5-trimethoxyallylbenzene). "
This Beeee has a theory ~7-12% of a bottle of Nutmeg oil distilled will give the precious MYRISTICIN
This Beeeee continued to read "The isomerization of this fraction with alcoholic potassium hydroxide yielded (trans) isomyristicin"
This Beeeee read from another weblink
https://www.erowid.org/archive/rhodium/chemistry/myristicinaldehyde.html
and another weblink https://www.erowid.org/library/books_online/pihkal/pihkal132.shtml
"The syntheses have actually started from myristicinaldehyde (3-methoxy- 4,5-methylenedioxybenzaldehyde) (IV) produced by isomerisation of myristicin to isomyristicin and subsequent oxidation"
This Beeee couldn't find a direct scientific layout of [Isomyristicin ---> Myristicinaldehyde]
...soooo... :/ ... however, SWIM came across a weblink "isosafrole to piperonal" that may be relative ... https://www.erowid.org/archive/rhodium/chemistry/piperonal.isosafrole.html
This Beeeeeee read from this link "
Benzaldehydes from Propenylbenzenes
by Station[ Back to the Chemistry Archive ]
Introduction
The preparation of aromatic aldehydes by the oxidation of compounds with side-chains containing ethylenic linkages via potassium permanganate and potassium dichromate was investigated, including the oxidation of isosafrole to piperonal and of isoeugenol to vanillin.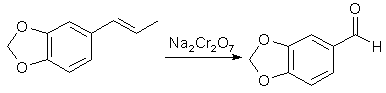
Since the initial materials are not completely miscible with water, the effect of introducing a dispersing agent was studied; for this purpose sulphanilic acid (which had the additional advantage of protecting the aldehyde when formed from further oxidation) and also Dispersol were used. The oxidation of isosafrole to piperonal by potassium permanganate under tested reaction conditions was far too vigorous, and the main product was piperonylic acid, whereas sodium dichromate and sulphuric acid afforded a 70% yield of piperonal, which was increased to 80% and 86.5% by the use of Dispersol and sulphanilic acid respectively as dispersing agents. The higher yield promoted by the sulphanilic acid was ascribed to ephemeral formation of a Schiff's base with the aldehyde when formed. With isoeugenol, in which the benzene structure is not immobilized by the clamping arrangement of the methylenedioxy group, the chromic acid oxidation gives a yield of 67% of vanillin as compared with the 86.5% of piperonal obtained from isosafrole.
Experimental: The preparation of aldehydes
(a) Without dispersing agent.A mixture of isosafrole (32.4g, 0.2 mol), 50% aqueous sulphuric acid (160 g) and water (1 L) at 30-40°C was vigorously stirred during the gradual addition over 30 minutes of a solution of sodium dichromate (44g, 0.13 mol, +10% excess) in water (200 ml). Reduction of the dichromate to green chromium salt appeared to be almost immediate; the mixture was twice extracted with benzene (600 ml), and the combined extracts were washed with 5% aqueous sodium hydroxide (200ml) followed by water (500ml). The extract was then dried over anhydrous calcium chloride, and the benzene removed, when the light brown residual oil crystallised on keeping (26g). The crude product was refluxed with ethyl alcohol (200 ml) and animal charcoal (10g), the crude solution filtered hot, and the bulk reduced to 100ml, when the piperonal was obtained in white crystals, mp 36-37°C (yield 21 g of pure product, 70%).
(b) With sulphanilic acid as dispersing agent.
Details were as in (a), except that the isosafrole was added to a solution of sulphanilic acid (12g) and sulphuric acid (80g, d 1.84) in water (1 L). Yield of pure product, 26 g. (86.5%). (c) Details were as in (a) except that Dispersol (5g) was present in the oxidation medium. Yield of pure product 24g (80%).
Experimental--Variations in temperature and in amount of oxidizing agent used:
| Variation | Yield |
| Standard experiment (above) | 86.5% |
| 50% Excess Na2Cr2O7 | 82.0% |
| Theoretical amount Na2Cr2O7 | 69.0% |
| Reaction conducted at 20°C | 78.5% |
| Reaction conducted at 20°C | 73.0% |
This Beeeee is sure Isomyristicin ----> Myristicinaldehyde
...
This Beeeee believes this weblink https://www.erowid.org/archive/rhodium/chemistry/myristicinaldehyde.shulgin.html
may be off topic
but [Isomyristicin ------> 1-(3-Methoxy-4,5-methylenedioxyphenyl)-2-nitropropene]
via tetranitromethane
This Beeeee continued reading this discourse " Experimental
1-(3-Methoxy-4,5-methylenedioxyphenyl)-2-nitropropene (2)
A solution of 50 g isomyristicin (1) in 300 ml dry acetone and 24 g pyridine was cooled to 0°C with vigorous stirring. There was then added 54 g of cold tetranitromethane which caused a slight temperature rise (to 5°C) despite the external cooling. The stirring was continued for 2 min at which time the reaction mixture was quenched with a chilled solution of 16.8 g KOH in 300 ml H2O. Stirring was continued while the heat of neutralization dissipated. and the product was then removed by filtration. An additional quantity of the nitropropene (for a total of 50.7 g, 82%) was obtained from the filtrates by extraction with methylene chloride. The low melting point (103°C) was due to a small contamination with myristicinaldehyde which is generated concurrently. Although it cannot interfere with the subsequent reaction below, it can be removed by recrystallization from methanol to yield yellow needles of 2, mp 110°C."
This Beeeee along with the nitropropene discourse, may be simularly conducted with Apiolealdehyde
"
Apiolealdehyde (4, R = OCH3)
Employing the procedure described above for isomyristicin, isoapiole (mp 55–56°C obtained from apiole of Oil of Parsley) was nitrated to 1-(2,5-dimethoxy-3,4 methylenedioxyphenyl)-2-nitropropene, and this intermediate was similarly converted to the aldehyde. This product aldehyde (mp 102–103°C) was obtained in a 75% overall yield from isoapiole."
This Beeeee had ended the off subject discourse and we continue with the
The MYRISTICIN ALDEHYDE.
This Beeeee came into conclusion of this 'aromatic aldehyde' is more rare than of its chemical brother Heliotropin, just guessing there wasn't any aromatherapy fragrance public usage ???
Too much extractions and scientific methods for public corporations to care to spend researching upon this very delicate rare "nutmeg aldehyde"???
Legitimate reason and usage for these special aldehydes ???
Any actual real photo pictures or videos of this aromatic aldehyde ???
What reason of the expensive price ???
What reason of so little scientific notes ???
*********?????????...
MAY SOMEBODY OUT THERE ADD SOME REFERENCES OR SCIENTIFIC LITERATURE
OF
PROPENYLBENZENE OXIDATION VIA potassium permanganate and sodium/potassium dichromate TO ALDEHYDE
...???????????************
??????????? Aromatic allylbenzene ------>
Aromatic aldehyde ????????
Soooo little is known!
This Beeeee believes conducting the propenylbenzene oxidation to the aromatic aldehyde would have more product and save lesssssss $ than to buy those few grams for atleast hundreds of dollars.
Yet Myristicinaldehyde, Piperonal, Apiolealdehyde,
2,4,5-trimethoxy-benzaldehyde (asarone-like),
3,4,5-trimethoxybenzaldehyde (elemicin -like) and more aldehydes have quite a curious fascination, potential for organic chemistry, and are indeed worth a look.
This Beeeee would like to thank the ladies and gentlemen for taking time to read about this interesting science phenomenal.
Thank you
REFERENCES for 3-Methoxy-4,5-methylenedioxybenzaldehyde (Myristicinaldehyde)
Product Link
Myristicin Aldehyde MSDS
5-Methoxypiperonal MSDS
"Nutmeg Benzaldehyde" Chemical & Synthesis Info

myristicin aldehyde
Chemsrc provides myristicin aldehyde(CAS#:5780-07-4) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Articles of myristicin aldehyde are included as well.
 www.chemsrc.com
www.chemsrc.com
A Total Synthesis of Myristicinaldehyde
A Total Synthesis of Myristicin
Benzaldehydes from Propenylbenzenes
Reference: Davies and Hodgson, J.S.C.I., June 1943, 91-93.
Alexander & Ann Shulgin
P.I.H.K.A.L.
#132 MMDA
3-METHOXY-4,5-METHYLENEDIOXYAMPHETAMINE
Alexander & Ann Shulgin
P.I.H.K.A.L.
#109 MDMA
MDM; ADAM; ECSTASY; 3,4-METHYLENEDIOXY-N-METHYLAMPHETAMINE