- Language
- 🇺🇸
- Joined
- May 10, 2025
- Messages
- 202
- Reaction score
- 29
- Points
- 28
Another off-topic discourse of an example of a
propenylbenzene isomer ---->
Aromatic nitropropene
P.I.H.K.A.L.
SYNTHESIS: Apiole, as the crystalline essential oil 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene, is isolated directly from commercial Oil of Parsley, by careful fractional distillation. It is the fraction that boils at 165-167 °C at 27 mm/Hg. A solution of 19.8 g apiole in a mixture of 43 g KOH and 60 mL hot EtOH was heated in the steam bath for 24 h. With vigorous stirring, it was diluted with H2O, at a rate which the crystals that formed spontaneously could accumulate from the turbidity that was generated. When no more H2O could be added (there was persistent oiling out of material) the reaction mixture was filtered to give 12.1 g of an amber solid material. This was recrystallized from 20 mL boiling hexane, which was filtered while hot to remove insolubles. From the cooled filtrate, there was obtained 9.3 g of 2,5-dimethoxy-3,4-methylenedioxy-1-propenylbenzene, isoapiole, as pale cream-colored solids.
A stirred solution of 8.8 g 2,5-dimethoxy-3,4-methylenedioxy-1-propenylbenzene and 3.9 g pyridine in 45 mL acetone was cooled to ice-bath temperatures, and treated with 7.9 g tetranitromethane. This extremely dark reac-tion was stirred at 0 °C for 5 min, then quenched with a solution of 2.6 g KOH in 45 mL H2O. With continued stirring, there appeared yellow crystals of 1-(2,5-dimethoxy-3,4-methylenedioxyphenyl)-2-nitropropene which, after filtering, washing with 50% acetone and air drying, weighed 8.0 g and had a mp of 110-111 °C.
The same procedure with Oil of Dill :
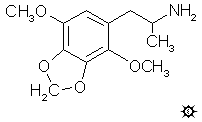
#59 DMMDA-2
2,3-DIMETHOXY-4,5-METHYLENEDIOXYAMPHETAMINE
[3D .mol structure]
DOSAGE: about 50 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 50 mg) I am into it; it is much like MDA.
EXTENSIONS AND COMMENTARY: This is pretty sparse information upon which to build a picture of biological activity. First, the synthesis was done by someone else and, as I have not been able to find where the notes are, this will be the one recipe in the footnote without explicit directions incorporated.
The procedure used was exactly the same as that described for DMMDA, except that the starting material was dillapiole rather than apiole. The dillapiole was obtained by the careful fractionation of Oil of Dill (as opposed to the isolation of apiole from the careful fractionation of Oil of Parsley). Isomerization to isodillapiole, nitration with tetra-nitromethane to give 1-(2,3-dimethoxy-4,5-methylenedioxyphenyl)-2-nitropropene, and its reduction with LAH in ether to give 2,3-dimethoxy-4,5-methylenedioxyamphetamine hydrochloride (DMMDA-2) proceeded in a precisely analogous manner to the preparation of DMMDA.
There's a Beeee going off-roading with a relative discourse
propenylbenzene isomer ---->
Aromatic nitropropene
P.I.H.K.A.L.
#58 DMMDA
2,5-DIMETHOXY-3,4-METHYLENEDIOXYAMPHETAMINESYNTHESIS: Apiole, as the crystalline essential oil 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene, is isolated directly from commercial Oil of Parsley, by careful fractional distillation. It is the fraction that boils at 165-167 °C at 27 mm/Hg. A solution of 19.8 g apiole in a mixture of 43 g KOH and 60 mL hot EtOH was heated in the steam bath for 24 h. With vigorous stirring, it was diluted with H2O, at a rate which the crystals that formed spontaneously could accumulate from the turbidity that was generated. When no more H2O could be added (there was persistent oiling out of material) the reaction mixture was filtered to give 12.1 g of an amber solid material. This was recrystallized from 20 mL boiling hexane, which was filtered while hot to remove insolubles. From the cooled filtrate, there was obtained 9.3 g of 2,5-dimethoxy-3,4-methylenedioxy-1-propenylbenzene, isoapiole, as pale cream-colored solids.
A stirred solution of 8.8 g 2,5-dimethoxy-3,4-methylenedioxy-1-propenylbenzene and 3.9 g pyridine in 45 mL acetone was cooled to ice-bath temperatures, and treated with 7.9 g tetranitromethane. This extremely dark reac-tion was stirred at 0 °C for 5 min, then quenched with a solution of 2.6 g KOH in 45 mL H2O. With continued stirring, there appeared yellow crystals of 1-(2,5-dimethoxy-3,4-methylenedioxyphenyl)-2-nitropropene which, after filtering, washing with 50% acetone and air drying, weighed 8.0 g and had a mp of 110-111 °C.
The same procedure with Oil of Dill :
#59 DMMDA-2
2,3-DIMETHOXY-4,5-METHYLENEDIOXYAMPHETAMINE
[3D .mol structure]
DOSAGE: about 50 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 50 mg) I am into it; it is much like MDA.
EXTENSIONS AND COMMENTARY: This is pretty sparse information upon which to build a picture of biological activity. First, the synthesis was done by someone else and, as I have not been able to find where the notes are, this will be the one recipe in the footnote without explicit directions incorporated.
The procedure used was exactly the same as that described for DMMDA, except that the starting material was dillapiole rather than apiole. The dillapiole was obtained by the careful fractionation of Oil of Dill (as opposed to the isolation of apiole from the careful fractionation of Oil of Parsley). Isomerization to isodillapiole, nitration with tetra-nitromethane to give 1-(2,3-dimethoxy-4,5-methylenedioxyphenyl)-2-nitropropene, and its reduction with LAH in ether to give 2,3-dimethoxy-4,5-methylenedioxyamphetamine hydrochloride (DMMDA-2) proceeded in a precisely analogous manner to the preparation of DMMDA.
There's a Beeee going off-roading with a relative discourse
- Language
- 🇺🇸
- Joined
- May 10, 2025
- Messages
- 202
- Reaction score
- 29
- Points
- 28
- Language
- 🇺🇸
- Joined
- May 10, 2025
- Messages
- 202
- Reaction score
- 29
- Points
- 28
* CAUTION ! TETRANITROMETHANE IS A DANGEROUS CHEMICAL !!!
MDA, JMC, 9, 445 (1966)
A solution of 6.5 g of isosafrole (or analog), 3.3 g of pyridine in 41 g of dry acetone is cooled
to 0°, Add 6.9 g of cold tetranitromethane over one min with good stirring. Stir for 2 more min
and quench as above with 2.2 g of KOH in 40 ml of water, add a little more water and extract
the nitropropene with dichloromethane. Evaporate after drying and recrystallize from methanol.
This extracting with dichloromethane should be used in the above formula to get an additional
amount of product from the filtrate. Follow the instructions immediately above. Dichloromethane
is the same as methylene chloride.
CAUTION: Tetranitromethane is nasty stuff, it explodes on contact with most any impurity, and
is toxic and corrosive. For these reasons it cannot be shipped UPS; it must be trucked and the
shipping will cost a minimum of $50. Even if you order 1 g you pay $50 shipping, so if you
are going to order some you may as well get as much as you can possibly afford. It is possible
to have a university or analytical lab make some for you, but the cost of these little outfits may
overcome the high shipping costs from the major suppliers. Expect to pay $35 for 5 g from the
major suppliers. If you live close enough to the big suppliers to pick it up in person, you are lucky;
if you have an accident on the way home with 50 g of the stuff in your car, you may be very
unlucky.
CAUTION
This next formula is easy, and although I have never seen the effects or potency on humans,
I believe that it would be at least as powerful as DOM, due to the substituted bromine atom.
As in most formulas, using nitromethane produces a product 10 times weaker than nitroethane;
use the one that suits you best.
***READ THE "CAUTION" about tetranitromethane ****
MDA, JMC, 9, 445 (1966)
A solution of 6.5 g of isosafrole (or analog), 3.3 g of pyridine in 41 g of dry acetone is cooled
to 0°, Add 6.9 g of cold tetranitromethane over one min with good stirring. Stir for 2 more min
and quench as above with 2.2 g of KOH in 40 ml of water, add a little more water and extract
the nitropropene with dichloromethane. Evaporate after drying and recrystallize from methanol.
This extracting with dichloromethane should be used in the above formula to get an additional
amount of product from the filtrate. Follow the instructions immediately above. Dichloromethane
is the same as methylene chloride.
CAUTION: Tetranitromethane is nasty stuff, it explodes on contact with most any impurity, and
is toxic and corrosive. For these reasons it cannot be shipped UPS; it must be trucked and the
shipping will cost a minimum of $50. Even if you order 1 g you pay $50 shipping, so if you
are going to order some you may as well get as much as you can possibly afford. It is possible
to have a university or analytical lab make some for you, but the cost of these little outfits may
overcome the high shipping costs from the major suppliers. Expect to pay $35 for 5 g from the
major suppliers. If you live close enough to the big suppliers to pick it up in person, you are lucky;
if you have an accident on the way home with 50 g of the stuff in your car, you may be very
unlucky.
CAUTION
This next formula is easy, and although I have never seen the effects or potency on humans,
I believe that it would be at least as powerful as DOM, due to the substituted bromine atom.
As in most formulas, using nitromethane produces a product 10 times weaker than nitroethane;
use the one that suits you best.
***READ THE "CAUTION" about tetranitromethane ****