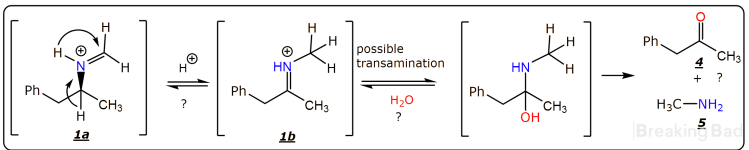The short answer is: under the reaction conditions, it is almost impossible to obtain any significant amount of
methamphetamine, starting from amphetamine. The only product is
N,N-dimethylamphetamine, together with the unreacted
amphetamine.
The reaction in question is Eschweiler–Clarke procedure, i.e. exhaustive methylation of primary (and secondary) amines, using formaldehyde and formic acid. This is an old, but highly efficient method for the preparation of tertiary
N,
N-dimethyl-alkyl amines.
It is unlikely that any significant amount of a secondary amine (i.e. methamphetamine) will be obtained under the reaction conditions, regardless of the stoichiometry. Secondary amines are generally more reactive than primary ones, so they rapidly react further, yielding tertiary amines. In addition, the reaction requires elevated temperatures (~100oC), further reducing the selectivity, and the possibility of secondary amine formation.
In the case when a limited amounts of formic acid and formaldehyde are used (any amount lower then stoichiometric), the typical composition of the reaction mixture should be as shown below:
Thus, the desired product
3 (dextromethamphetamine or racemic methamphetamine) will be absent, almost certainly. (Any experimental analysis of the reaction mixture requires, at least, gas chromatography, preferably coupled gas chromatography-mass spectrometry).
Furthermore, even if some methamphetamine is produced, the mixture (
1 +
2 +
3) would be almost impossible to separate on a preparative scale (e.g. >1 g), due to similar boiling points and other characteristics (all three amines are fairly volatile, practically precluding column chromatography). The only choice would be preparative gas chromatography or preparative HPLC, both extremely expensive for the compounds in question. Thus, the whole experiment would be practically useless.
Mechanistically, the reaction involves the hydride transfer from formic acid, as shown bellow:
The question concerning the racemization is more complex, however it is relevant only to the tertiary amine
2. The possible racemization can occur, via the acid-catalyzed equilibrium of imines
1a and
1b, shown bellow. Also, transamination is possible, leading to ketone
4 and methylamine
5. All those reactions are a possibility only, not likely to proceed to any significant extent.
Thus, it is reasonable to expect
N,
N-dimethyl amine
2 in good yields as the only product, likely with little or no racemization.
Generally, the selective conversion of primary amines to the secondary ones (e.g. amphetamine to methamphetamine) requires different synthetic approaches. For example, reduction of secondary formamides with LiAlH4 or DIBAL-H, or alternatively,
N-alkylation of amidate anions, derived from secondary carboxamides, such as formamides, or BOC carbonates, followed by acid hydrolysis. Some other methods exist as well.
Finally, methamphetamine (dextro or racemic) is not normally prepared from amphetamine on any significant scale, due to the relative complexity of the procedures and low cost-effectiveness. However, it is not impossible, albeit not using Eschweiler–Clarke procedure.