WillD
Expert
- Joined
- Jul 19, 2021
- Messages
- 774
- Reaction score
- 1,054
- Points
- 93
Reaction scheme:
 Equipment and glassware:
Equipment and glassware:
Reagents:
We recommend buying a ready-made precursor, which is quite difficult to find. According to our information, this substance is available in brumer.com
- 50 L Batch reactor, which is equipped with drip funnel, top stirrer, thermometer, temperature control system (cooling) and reflux condenser;
- Funnel;
- Several buckets 10 and 20 L;
- Vacuum source;
- Laboratory scale (1-2000 g is suitable);
- Measuring cylinder 1000 mL;
- Glass rod (big one) and spatula;
- Nutsche filter;
- Rotary evaporator;
- Scoops;
Reagents:
- Tin (IV) chloride (SnCl4) 2000 ml (cas 7646-78-8);
- Dichloromethane (CH2Cl2) 15 l;
- Indole 1000 g (cas 120-72-9);
- 2,2,3,3-Tetramethylcyclopropanecarbonyl chloride 1371 g (cas 24303-61-5); Supplier
- Ice distilled water 20 l;
- Ethyl acetate 15 l;
- Sodium sulfate Na2SO4 (or MgSO4) anhydrous ~ 2 kg;
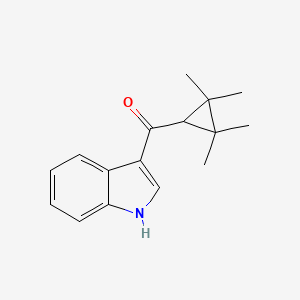
Synthesis:1. Tin (IV) chloride (SnCl4) 2000 ml was added in one portion into a reaction mixture of dichloromethane (CH2Cl2) 15 l with indole 1000 g. Mixture is stirred in a 50 L batch reactor with cooling.
2. Next, reactor cooling is removed and reaction mixture is stirred for 30 min at room temperature. 2,2,3,3-Tetramethylcyclopropanecarbonyl chloride 1371 g (cas 24303-61-5) in small portions and then nitromethane 10 l are added.
3. The mixture is stirred for 2 h at room temperature.
4. Reaction mixture is quenched with ice water 20 l, filtered from inorganic precipitates via Nutsche filter. Organic substances are extracted with ethyl acetate 15 l.
5. The organic extract is dried over Na2SO4 (or MgSO4) and concentrated at reduced pressure in rotary evaporator to give the product as a crystals.
UR-144, A-834,735, XLR-11 can be obtained from TMCP-indole intermediate.
The synthesis of this substance is quite complex.2. Next, reactor cooling is removed and reaction mixture is stirred for 30 min at room temperature. 2,2,3,3-Tetramethylcyclopropanecarbonyl chloride 1371 g (cas 24303-61-5) in small portions and then nitromethane 10 l are added.
3. The mixture is stirred for 2 h at room temperature.
4. Reaction mixture is quenched with ice water 20 l, filtered from inorganic precipitates via Nutsche filter. Organic substances are extracted with ethyl acetate 15 l.
5. The organic extract is dried over Na2SO4 (or MgSO4) and concentrated at reduced pressure in rotary evaporator to give the product as a crystals.
UR-144, A-834,735, XLR-11 can be obtained from TMCP-indole intermediate.
We recommend buying a ready-made precursor, which is quite difficult to find. According to our information, this substance is available in brumer.com
Last edited by a moderator: