- Language
- 🇺🇸
- Joined
- Feb 26, 2023
- Messages
- 53
- Reaction score
- 43
- Points
- 18
This article provides you with a low-cost, mild condition, high-yield P2P synthesis process, and the conditions are easy to scale.It's just that I don't know if the raw material aniline is regulated in other countries, but it is quite cheap in my country, only about 1 US dollar per kilogram.
The process design requires the use of some advanced instruments, which may not be friendly to family workshops, and it is recommended that everyone improve it by themselves.
Process introduction:
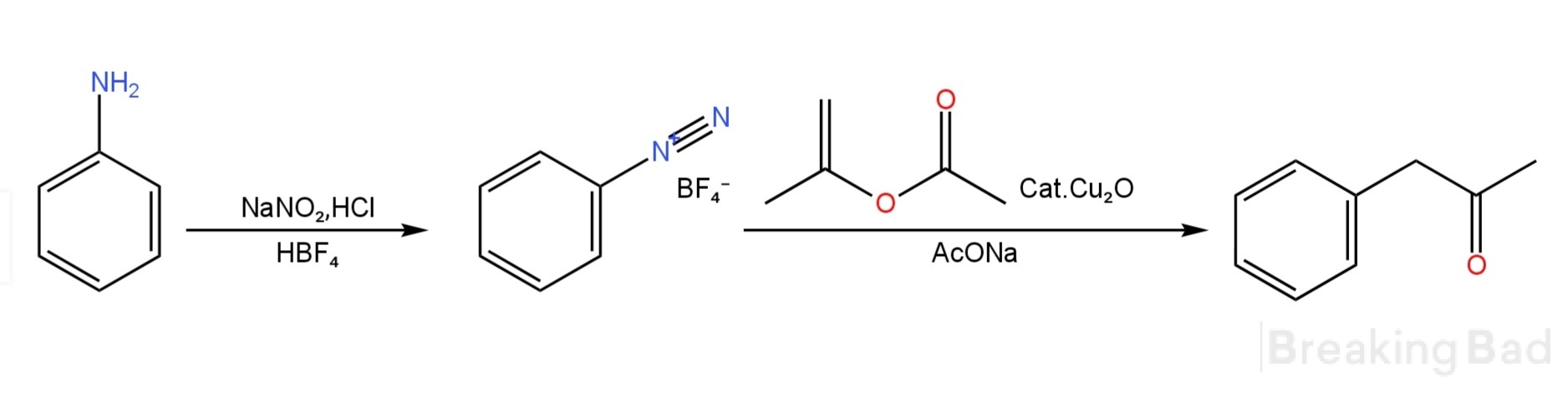
This process uses aniline and sodium nitrite in the presence of hydrochloric acid to prepare diazonium benzene through diazotization reaction, and uses tetrafluoroboric acid (HBF4) to salt diazobenzene to obtain solid diazobenzene tetrafluoroborate. In the presence of anhydrous sodium acetate (AcONa), diazobenzene tetrafluoroborate is catalyzed by cuprous oxide (Cu2O), and reacts with isopropenyl acetate (IPA) without solvent to prepare 1-phenyl-2-acetone.
Because my English is not very good, this article is translated by Google, please forgive me if the grammar is not smooth.
Practice steps:
Manufacture of phenyldiazonium tetrafluoroborate:
-If your aniline is yellow, please add a small amount of zinc powder to the aniline and use it after distillation.
-A 2000ml device.
-Add 450.6ml37% hydrochloric acid and 652.18ml ultrapure water, stir.
-Add 165.54g of aniline,keep stirring.
-Cool down to below 5°C.
-Add a solution made of 125.69g of anhydrous sodium nitrite and 166.01ml of ultrapure water dropwise under stirring, and keep the temperature below 5°C.
-After the addition, the temperature was kept at 3°C to 5°C and the reaction was stirred for 40 minutes.
-Dissolve 142.77g of anhydrous boric acid (H3BO3) in 462.45927ml of 40% hydrofluoric acid (HF-H2Oaq40%) to make tetrafluoroboric acid, keep stirring at 3°C to 5°C and add to the device.*1
-Turn off the stirring and keep the temperature for 2 hours.
-Suction filter the reaction mixture.
-Use these solvents in turn to rinse the solids in the funnel: 80ml of tetrafluoroboric acid produced by the above method diluted to a volume of 356ml, 356ml of 50% ethanol, 356ml of 95% ethanol, two parts of 356ml of anhydrous ether.
-Dry the solid in a freeze dryer or use a vacuum oven at 36 °C.
-Obtain white powdery diazonium tetrafluoroborate phenyl salt, the yield is about 90%.
Preparation of 1-phenyl-2-propanone (P2P):
-A 2000ml device.
-Add anhydrous sodium acetate (AcONa) 528.81g, cuprous oxide (Cu2O) 33.90g, isopropenyl acetate (IPA) 1423.73ml, stir.
-Keep stirring, add 447.46g of the diazonium tetrafluoroborate solid prepared in the previous step at room temperature, and control the temperature not to exceed 40°C.
-After the addition, keep the temperature at 37°C to 38°C, and stir for 6 hours.
-Suction filter the reaction mixture.
-Rinse the solids in the funnel with 250 mL of dichloromethane (CH2Cl2) and combine into the filtrate.
-Evaporate dichloromethane using a rotary evaporator.
-The remaining liquid is distilled under reduced pressure, and the fraction from 0.27kPa/70°C to 72°C is collected under the monitoring of a Pirani vacuum gauge.
-Obtain 1-phenyl-2-propanone (P2P), the yield is about 91%.
Precautions:
-*1:The process of dissolving anhydrous boric acid in 40% hydrofluoric acid to produce tetrafluoroboric acid is very exothermic, which can cause the solution to bump. It is recommended to pre-freeze 40% hydrofluoric acid and pay attention to the temperature when mixing. In addition, this operation must not be carried out with any glass container, otherwise your container will be corroded and dissolved and release highly toxic gas, you can use any plastic container for the preparation of tetrafluoroboric acid.
-The diazonium tetrafluoroborate phenyl salt prepared in the first step reaction will deteriorate within a day or two if placed in the air. If it needs to be stored for a long time, please seal it in a container, replace the nitrogen or argon atmosphere, and place it in the refrigerator. Store at a temperature below -20°C.
-In both reactions the viscosity is too high for the magnetic stirrer to work, please use an overhead stirrer for stirring.
Compound data:
1-phenyl-2-propanone 1H-NMR δ:2.14(s,3H,CH3),3.69(s,2H,CH2),7.19(s,1H,PhH),7.28(s,1H,PhH),7.31(s,1H,PhH),7.33(s,1H,PhH),7.34(s,1H,PhH)
1-phenyl-2-propanone 13C-NMR δ:29.23(CH3),51.05(CH2),206.36(CO),134.31,129.41,128.76,127.08
The process design requires the use of some advanced instruments, which may not be friendly to family workshops, and it is recommended that everyone improve it by themselves.
Process introduction:
This process uses aniline and sodium nitrite in the presence of hydrochloric acid to prepare diazonium benzene through diazotization reaction, and uses tetrafluoroboric acid (HBF4) to salt diazobenzene to obtain solid diazobenzene tetrafluoroborate. In the presence of anhydrous sodium acetate (AcONa), diazobenzene tetrafluoroborate is catalyzed by cuprous oxide (Cu2O), and reacts with isopropenyl acetate (IPA) without solvent to prepare 1-phenyl-2-acetone.
Because my English is not very good, this article is translated by Google, please forgive me if the grammar is not smooth.
Practice steps:
Manufacture of phenyldiazonium tetrafluoroborate:
-If your aniline is yellow, please add a small amount of zinc powder to the aniline and use it after distillation.
-A 2000ml device.
-Add 450.6ml37% hydrochloric acid and 652.18ml ultrapure water, stir.
-Add 165.54g of aniline,keep stirring.
-Cool down to below 5°C.
-Add a solution made of 125.69g of anhydrous sodium nitrite and 166.01ml of ultrapure water dropwise under stirring, and keep the temperature below 5°C.
-After the addition, the temperature was kept at 3°C to 5°C and the reaction was stirred for 40 minutes.
-Dissolve 142.77g of anhydrous boric acid (H3BO3) in 462.45927ml of 40% hydrofluoric acid (HF-H2Oaq40%) to make tetrafluoroboric acid, keep stirring at 3°C to 5°C and add to the device.*1
-Turn off the stirring and keep the temperature for 2 hours.
-Suction filter the reaction mixture.
-Use these solvents in turn to rinse the solids in the funnel: 80ml of tetrafluoroboric acid produced by the above method diluted to a volume of 356ml, 356ml of 50% ethanol, 356ml of 95% ethanol, two parts of 356ml of anhydrous ether.
-Dry the solid in a freeze dryer or use a vacuum oven at 36 °C.
-Obtain white powdery diazonium tetrafluoroborate phenyl salt, the yield is about 90%.
Preparation of 1-phenyl-2-propanone (P2P):
-A 2000ml device.
-Add anhydrous sodium acetate (AcONa) 528.81g, cuprous oxide (Cu2O) 33.90g, isopropenyl acetate (IPA) 1423.73ml, stir.
-Keep stirring, add 447.46g of the diazonium tetrafluoroborate solid prepared in the previous step at room temperature, and control the temperature not to exceed 40°C.
-After the addition, keep the temperature at 37°C to 38°C, and stir for 6 hours.
-Suction filter the reaction mixture.
-Rinse the solids in the funnel with 250 mL of dichloromethane (CH2Cl2) and combine into the filtrate.
-Evaporate dichloromethane using a rotary evaporator.
-The remaining liquid is distilled under reduced pressure, and the fraction from 0.27kPa/70°C to 72°C is collected under the monitoring of a Pirani vacuum gauge.
-Obtain 1-phenyl-2-propanone (P2P), the yield is about 91%.
Precautions:
-*1:The process of dissolving anhydrous boric acid in 40% hydrofluoric acid to produce tetrafluoroboric acid is very exothermic, which can cause the solution to bump. It is recommended to pre-freeze 40% hydrofluoric acid and pay attention to the temperature when mixing. In addition, this operation must not be carried out with any glass container, otherwise your container will be corroded and dissolved and release highly toxic gas, you can use any plastic container for the preparation of tetrafluoroboric acid.
-The diazonium tetrafluoroborate phenyl salt prepared in the first step reaction will deteriorate within a day or two if placed in the air. If it needs to be stored for a long time, please seal it in a container, replace the nitrogen or argon atmosphere, and place it in the refrigerator. Store at a temperature below -20°C.
-In both reactions the viscosity is too high for the magnetic stirrer to work, please use an overhead stirrer for stirring.
Compound data:
1-phenyl-2-propanone 1H-NMR δ:2.14(s,3H,CH3),3.69(s,2H,CH2),7.19(s,1H,PhH),7.28(s,1H,PhH),7.31(s,1H,PhH),7.33(s,1H,PhH),7.34(s,1H,PhH)
1-phenyl-2-propanone 13C-NMR δ:29.23(CH3),51.05(CH2),206.36(CO),134.31,129.41,128.76,127.08
