You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Cocaine synthesis
- Thread starter theone
- Start date
I found this awhile back. It's synthesis of cocaine using tropinone which I believe is commercially available.
I copied and pasted.
================================================================= This file is a part of the 1999 Hyperreal Drug Archives Snapshot. This snapshot is hosted by Erowid and will not be updated after October 1999. The information in these files may be out of date. See Erowid's Psychoactive Vaults for more current info. ================================================================= From: [email protected] (Jon Taylor) Newsgroups: alt.drugs Subject: Cocaine Synthesis Date: 18 Apr 1994 18:30:40 -0600 Message-ID: <2ov8ng$[email protected]> Enjoy! -Jon CUT HERE /\/\/\/\/\/\/\/\/\/\/\/\ Cocaine Synthesis Scanned From _Recreational Drugs: A Complete Guide to Manufacturing_ COCAINE Although this drug is categorized as a local anesthetic, I have chosen to put it in with the hallucinogens because of the psycho- tomimetic effects that it produces. Cocaine is not a phenylethyl- amine, but it produces central nervous system arousal or stimulant effects which closely resemble those of the amphetamines, the methylenedioxyamphetamines in particular. This is due to the inhibition by cocaine of re-uptake of the norepinepherine released by the adrenergic nerve terminals, leading to an enhanced adrenergic stimulation of norepinephrine receptors. The increased sense of well being and intense, but short lived, euphoric state produced by cocaine requires frequent administration. Cocaine does not penetrate the intact skin, but is readily absorbed from the mucus membranes, creating the need to snort it. This accounts for the ulceration of the nasal septum after cocaine has been snorted for long periods. The basic formula for cocaine starts by purchasing or making tropinone, converting the tropinone into 2-carbomethoxytropinone (also known as methyl-tropan-3-one-2-carboxylate), reducing this to ecgonine, and changing that to cocaine. Sounds easy? It really is not very simple, but with Reagan's new drug policies, cracking down on all of the drug smuggling at the borders, this synthetic cocaine may be the source of the future. This synthesis is certainly worth performing with the high prices that cocaine is now commanding. As usual, I will start with the precursors and intermediates leading up to the product. Succindialdehyde. This can be purchased, too. 23.2 g of succinaldoxime powder in 410 ml of 1 N sulfuric acid and add dropwise with stirring at 0Ą a solution of 27.6 g of sodium nitrite in 250 ml of water over 3 hours. After the addition, stir and let the mixture rise to room temp for about 2 hours, taking care not to let outside air into the reaction. Stir in 5 g of Ba carbonate and filter. Extract the filtrate with ether and dry, evaporate in vacuo to get the succindialdehyde. This was taken from JOC, 22, 1390 (1957). To make succinaldoxime, see JOC, 21, 644 (1956). Complete Synthesis of Succindialdehyde. JACS, 68, 1608 (1946). In a 2 liter 3 necked flask equipped with a stirrer, reflux condenser, and an addition funnel, is mixed 1 liter of ethanol, 67 g of freshly distilled pyrrole, and 141 g of hydroxylamine hydrochloride. Heat to reflux until dissolved, add 106 g of anhydrous sodium carbonate in small portions as fast as reaction will allow. Reflux for 24 hours and filter the mixture. Evaporate the filtrate to dryness under vacuo. Take up the residue in the minimum amount of boiling water, decolorize with carbon, filter and allow to recrystallize in refrigerator. Filter to get product and concentrate to get additional crop. Yield of succinaldoxime powder is a little over 40 g, mp is 171-172Ą. 5.8 g of the above powder is placed in a beaker of 250 ml capacity and 54 ml of 10% sulfuric acid is added. Cool to 0Ą and add in small portions of 7 g of sodium nitrite (if you add the nitrite too fast, nitrogen dioxide fumes will evolve). After the dioxime is completely dissolved, allow the solution to warm to 20Ą and effervescence to go to completion. Neutralize the yellow solution to litmus by adding small portions of barium carbonate. Filter off the barium sulfate that precipitates. The filtrate is 90% pure succindialdehyde and is not purified further for the reaction to create tropinone. Do this procedure 3 more times to get the proper amount for the next step, or multiply the amounts given by four and proceed as described above. Take the total amount of succinaldehyde (obtained from 4 of the above syntheses combined) and without further treatment or purification (this had better be 15.5 g of succindialdehyde) put into an Erlenmeyer flask of 4-5 liters capacity. Add 21.6 g of methylamine hydrochloride, 46.7 g of acetonedicarboxylic acid, and enough water to make a total volume of 2 liters. Adjust the pH to 8-10 by slowly adding a saturated solution of disodium phosphate. The condensate of this reaction (allow to set for about 6 days) is extracted with ether, the ethereal solution is dried over sodium sulphate and distilled, the product coming over at 113Ą at 25 mm of pressure is collected. Upon cooling, 14 g of tropinone crystallizes in the pure state. Tropinone can also be obtained by oxidation of tropine with potassium dichromate, but I could not find the specifics for this operation. 2-Carbomethoxytropinone. A mixture of 1.35 g of sodium methoxide (this is sodium in a minimum amount of methanol), 3.5 g of tropinone, 4 ml of dimethylcarbonate and 10 ml of toluene is refluxed for 30 min. Coo] to 0Ą and add 15 ml of water that contains 2.5 g of ammonium chloride. Extract the solution after shaking with four 50 ml portions of chloroform, dry, evaporate the chloroform in vacuo. Dissolve the oil residue in 100 ml of ether, wash twice with a mixture of 6 ml of saturated potassium carbonate and three ml of 3 N KOH. Dry and evaporate in vacuo to recover the unreacted tropinone. Take up the oil in a solution of aqueous ammonium chloride and extract with chloroform, dry, and evaporate in vacuo to get an oil. The oil is dissolved in hot acetone, cool, and scratch inside of flask with glass rod to precipitate 2- carbomethoxytropinone. Recrystallize 16 g of this product in 30 ml of hot methyl acetate and add 4 ml of cold water and 4 ml of acetone. Put in freezer for 2l/2 to 3 hours. Filter and wash the precipitate with cold methyl acetate to get pure product. Methylecgonine. 0.4 mole of tropinone is suspended in 80 ml of ethanol in a Parr hydrogenation flask (or something that can take 100 psi and not react with the reaction, like stainless steel or glass). 10 g of Raney Nickle is added with good agitation (stirring or shaking) followed by 2- 3 ml of 20% NaOH solution. Seal vessel, introduce 50 psi of hydrogen atmosphere (after flushing vessel with hydrogen) and heat to 40-50Ą. After no more uptake of hydrogen (pressure gauge will hold steady after dropping to its lowest point) bleed off pressure and filter the nickle off, rinse out bottle with chloroform and use this rinse to rinse off the nickle while still on the filter paper. Make the filtrate basic with KOH after cooling to 10Ą. Extract with chloroform dry, and evaporate the chloroform in vacuo to get an oil. Mix the oil plus any precipitate with an equal volume of dry ether and filter. Add more dry ether to the filtrate until no more precipitate forms, filter and add to the rest of the precipitate. Recrystallize from isopropanol to get pure methylecgonine. Test for activity. If active, skip down to the step for cocaine. If not active, proceed as follows. Stir with activated carbon for 30 min, filter, evaporate in vacuo, dissolve the brown liquid in methanol, and neutralize with 10% HCI acid in dry ether. Evaporate the ether until the two layers disappear, and allow to stand for 2 hours at 0Ą to precipitate the title product. There are many ways to reduce 2-carbomethoxytropinone to methylecgonine. I chose to design a Raney Nickle reduction because it is cheap and not as suspicious as LAH and it is much easier than zinc or sodium amalgams. Cocaine. 4.15 g of methylecgonine and 5.7 g of benzoic anhydride in 150 ml of dry benzene are gently refluxed for 4 hours taking precaution against H20 in the air (drying tube). Cool in an ice bath, acidify carefully with hydrochloric acid, dry, and evaporate in a vacuum to get a red oil which is treated with a little portion of isopropanoi to precipitate cocaine. As you can see, this is quite a chore. The coca leaves give ecgonine, which as you can see, is only a Jump away from cocaine. If you can get egconine, then dissolve 8l/2 g of it in 100 ml of ethanol and pass (bubble) dry HC1 gas through this solution for 30 min. Let cool to room temp and let stand for another 11/2 hours. Gently reflux for 30 min and evaporate in vacuo. Basify the residue oil with NaOH and filter to get 8.4 g of methylecgonine, which is converted to cocaine as in the cocaine step above. Below is given a somewhat easier method of producing tropinone by the general methods of Willstatter, who was instrumental in the first synthetic production of cocaine and several other alkaloids. After reviewing this method, I found it to be simpler than the above in many respects. Tropinone. 10 g of pyrrolidinediethyl diacetate are heated with 10 g of cymene and 2 g of sodium powder, the reaction taking place at about 160Ą. During the reaction (which is complete in about 10 min) the temp should not exceed 172Ą. The resulting reaction product is dissolved in water, then saturated with potassium carbonate, and the oil, which separates, is boiled with dilute sulfuric acid. 2.9 g of tropinone picrate forms and is filtered. Here are two more formulas devised by Willstatter that produce tropinone from tropine. Take note of the yield differences. Tropinone. To a solution of 25 g tropine, dissolved in 10 times its weight of 20% sulfuric acid are added 25 g of a 4% solution of potassium permanganate in 2 or 3 g portions over 45 min while keeping the temp at 10-12Ą. The addition of permanganate will cause heat (keep the temp 10-12Ą) and precipitation of manganese dioxide. The reaction mixture is complete in I hour. A large excess of NaOH is added and the reaction is steam distilled until I liter of distillate has been collected. The tropinone is isolated as the dibenzal compound by mixing the distillate with 40 g of benzaldehyde in 500 cc of alcohol and 40 g of 10% sodium hydroxide solution. Let stand several days to get dibenzaltropinone as yellow needles. Yield: 15.5 g, 28%. Recrystallize from ethanol to purify. Tropinone. A solution of 12 g of chromic acid in the same amount of water (12 g) and 60 g of glacial acetic acid is added dropwise with stirring over a period of 4 hours to a solution of 25 g of tropine in 500 cc of glacial acetic acid that has been warmed to 60-70Ą and is maintained at this temp during the addition. Heat the mixture for a short time on a steam bath until all the chromic acid has disappeared, cool and make strongly alkaline with NaOH. Extract with six 500 cc portions of ether and evaporate the ether in vacuo to get an oil that crystallizes readily. Purify by converting to the picrate or fractionally distill, collecting the fraction at 224-225Ą at 714 mm vacuo. The tropinones can be used in the above formula (or in a formula that you have found elsewhere) to be converted to cocaine. Remember to recrystallize the 2-carbomethoxytropinone before converting to methylecgonine.
I copied and pasted.
================================================================= This file is a part of the 1999 Hyperreal Drug Archives Snapshot. This snapshot is hosted by Erowid and will not be updated after October 1999. The information in these files may be out of date. See Erowid's Psychoactive Vaults for more current info. ================================================================= From: [email protected] (Jon Taylor) Newsgroups: alt.drugs Subject: Cocaine Synthesis Date: 18 Apr 1994 18:30:40 -0600 Message-ID: <2ov8ng$[email protected]> Enjoy! -Jon CUT HERE /\/\/\/\/\/\/\/\/\/\/\/\ Cocaine Synthesis Scanned From _Recreational Drugs: A Complete Guide to Manufacturing_ COCAINE Although this drug is categorized as a local anesthetic, I have chosen to put it in with the hallucinogens because of the psycho- tomimetic effects that it produces. Cocaine is not a phenylethyl- amine, but it produces central nervous system arousal or stimulant effects which closely resemble those of the amphetamines, the methylenedioxyamphetamines in particular. This is due to the inhibition by cocaine of re-uptake of the norepinepherine released by the adrenergic nerve terminals, leading to an enhanced adrenergic stimulation of norepinephrine receptors. The increased sense of well being and intense, but short lived, euphoric state produced by cocaine requires frequent administration. Cocaine does not penetrate the intact skin, but is readily absorbed from the mucus membranes, creating the need to snort it. This accounts for the ulceration of the nasal septum after cocaine has been snorted for long periods. The basic formula for cocaine starts by purchasing or making tropinone, converting the tropinone into 2-carbomethoxytropinone (also known as methyl-tropan-3-one-2-carboxylate), reducing this to ecgonine, and changing that to cocaine. Sounds easy? It really is not very simple, but with Reagan's new drug policies, cracking down on all of the drug smuggling at the borders, this synthetic cocaine may be the source of the future. This synthesis is certainly worth performing with the high prices that cocaine is now commanding. As usual, I will start with the precursors and intermediates leading up to the product. Succindialdehyde. This can be purchased, too. 23.2 g of succinaldoxime powder in 410 ml of 1 N sulfuric acid and add dropwise with stirring at 0Ą a solution of 27.6 g of sodium nitrite in 250 ml of water over 3 hours. After the addition, stir and let the mixture rise to room temp for about 2 hours, taking care not to let outside air into the reaction. Stir in 5 g of Ba carbonate and filter. Extract the filtrate with ether and dry, evaporate in vacuo to get the succindialdehyde. This was taken from JOC, 22, 1390 (1957). To make succinaldoxime, see JOC, 21, 644 (1956). Complete Synthesis of Succindialdehyde. JACS, 68, 1608 (1946). In a 2 liter 3 necked flask equipped with a stirrer, reflux condenser, and an addition funnel, is mixed 1 liter of ethanol, 67 g of freshly distilled pyrrole, and 141 g of hydroxylamine hydrochloride. Heat to reflux until dissolved, add 106 g of anhydrous sodium carbonate in small portions as fast as reaction will allow. Reflux for 24 hours and filter the mixture. Evaporate the filtrate to dryness under vacuo. Take up the residue in the minimum amount of boiling water, decolorize with carbon, filter and allow to recrystallize in refrigerator. Filter to get product and concentrate to get additional crop. Yield of succinaldoxime powder is a little over 40 g, mp is 171-172Ą. 5.8 g of the above powder is placed in a beaker of 250 ml capacity and 54 ml of 10% sulfuric acid is added. Cool to 0Ą and add in small portions of 7 g of sodium nitrite (if you add the nitrite too fast, nitrogen dioxide fumes will evolve). After the dioxime is completely dissolved, allow the solution to warm to 20Ą and effervescence to go to completion. Neutralize the yellow solution to litmus by adding small portions of barium carbonate. Filter off the barium sulfate that precipitates. The filtrate is 90% pure succindialdehyde and is not purified further for the reaction to create tropinone. Do this procedure 3 more times to get the proper amount for the next step, or multiply the amounts given by four and proceed as described above. Take the total amount of succinaldehyde (obtained from 4 of the above syntheses combined) and without further treatment or purification (this had better be 15.5 g of succindialdehyde) put into an Erlenmeyer flask of 4-5 liters capacity. Add 21.6 g of methylamine hydrochloride, 46.7 g of acetonedicarboxylic acid, and enough water to make a total volume of 2 liters. Adjust the pH to 8-10 by slowly adding a saturated solution of disodium phosphate. The condensate of this reaction (allow to set for about 6 days) is extracted with ether, the ethereal solution is dried over sodium sulphate and distilled, the product coming over at 113Ą at 25 mm of pressure is collected. Upon cooling, 14 g of tropinone crystallizes in the pure state. Tropinone can also be obtained by oxidation of tropine with potassium dichromate, but I could not find the specifics for this operation. 2-Carbomethoxytropinone. A mixture of 1.35 g of sodium methoxide (this is sodium in a minimum amount of methanol), 3.5 g of tropinone, 4 ml of dimethylcarbonate and 10 ml of toluene is refluxed for 30 min. Coo] to 0Ą and add 15 ml of water that contains 2.5 g of ammonium chloride. Extract the solution after shaking with four 50 ml portions of chloroform, dry, evaporate the chloroform in vacuo. Dissolve the oil residue in 100 ml of ether, wash twice with a mixture of 6 ml of saturated potassium carbonate and three ml of 3 N KOH. Dry and evaporate in vacuo to recover the unreacted tropinone. Take up the oil in a solution of aqueous ammonium chloride and extract with chloroform, dry, and evaporate in vacuo to get an oil. The oil is dissolved in hot acetone, cool, and scratch inside of flask with glass rod to precipitate 2- carbomethoxytropinone. Recrystallize 16 g of this product in 30 ml of hot methyl acetate and add 4 ml of cold water and 4 ml of acetone. Put in freezer for 2l/2 to 3 hours. Filter and wash the precipitate with cold methyl acetate to get pure product. Methylecgonine. 0.4 mole of tropinone is suspended in 80 ml of ethanol in a Parr hydrogenation flask (or something that can take 100 psi and not react with the reaction, like stainless steel or glass). 10 g of Raney Nickle is added with good agitation (stirring or shaking) followed by 2- 3 ml of 20% NaOH solution. Seal vessel, introduce 50 psi of hydrogen atmosphere (after flushing vessel with hydrogen) and heat to 40-50Ą. After no more uptake of hydrogen (pressure gauge will hold steady after dropping to its lowest point) bleed off pressure and filter the nickle off, rinse out bottle with chloroform and use this rinse to rinse off the nickle while still on the filter paper. Make the filtrate basic with KOH after cooling to 10Ą. Extract with chloroform dry, and evaporate the chloroform in vacuo to get an oil. Mix the oil plus any precipitate with an equal volume of dry ether and filter. Add more dry ether to the filtrate until no more precipitate forms, filter and add to the rest of the precipitate. Recrystallize from isopropanol to get pure methylecgonine. Test for activity. If active, skip down to the step for cocaine. If not active, proceed as follows. Stir with activated carbon for 30 min, filter, evaporate in vacuo, dissolve the brown liquid in methanol, and neutralize with 10% HCI acid in dry ether. Evaporate the ether until the two layers disappear, and allow to stand for 2 hours at 0Ą to precipitate the title product. There are many ways to reduce 2-carbomethoxytropinone to methylecgonine. I chose to design a Raney Nickle reduction because it is cheap and not as suspicious as LAH and it is much easier than zinc or sodium amalgams. Cocaine. 4.15 g of methylecgonine and 5.7 g of benzoic anhydride in 150 ml of dry benzene are gently refluxed for 4 hours taking precaution against H20 in the air (drying tube). Cool in an ice bath, acidify carefully with hydrochloric acid, dry, and evaporate in a vacuum to get a red oil which is treated with a little portion of isopropanoi to precipitate cocaine. As you can see, this is quite a chore. The coca leaves give ecgonine, which as you can see, is only a Jump away from cocaine. If you can get egconine, then dissolve 8l/2 g of it in 100 ml of ethanol and pass (bubble) dry HC1 gas through this solution for 30 min. Let cool to room temp and let stand for another 11/2 hours. Gently reflux for 30 min and evaporate in vacuo. Basify the residue oil with NaOH and filter to get 8.4 g of methylecgonine, which is converted to cocaine as in the cocaine step above. Below is given a somewhat easier method of producing tropinone by the general methods of Willstatter, who was instrumental in the first synthetic production of cocaine and several other alkaloids. After reviewing this method, I found it to be simpler than the above in many respects. Tropinone. 10 g of pyrrolidinediethyl diacetate are heated with 10 g of cymene and 2 g of sodium powder, the reaction taking place at about 160Ą. During the reaction (which is complete in about 10 min) the temp should not exceed 172Ą. The resulting reaction product is dissolved in water, then saturated with potassium carbonate, and the oil, which separates, is boiled with dilute sulfuric acid. 2.9 g of tropinone picrate forms and is filtered. Here are two more formulas devised by Willstatter that produce tropinone from tropine. Take note of the yield differences. Tropinone. To a solution of 25 g tropine, dissolved in 10 times its weight of 20% sulfuric acid are added 25 g of a 4% solution of potassium permanganate in 2 or 3 g portions over 45 min while keeping the temp at 10-12Ą. The addition of permanganate will cause heat (keep the temp 10-12Ą) and precipitation of manganese dioxide. The reaction mixture is complete in I hour. A large excess of NaOH is added and the reaction is steam distilled until I liter of distillate has been collected. The tropinone is isolated as the dibenzal compound by mixing the distillate with 40 g of benzaldehyde in 500 cc of alcohol and 40 g of 10% sodium hydroxide solution. Let stand several days to get dibenzaltropinone as yellow needles. Yield: 15.5 g, 28%. Recrystallize from ethanol to purify. Tropinone. A solution of 12 g of chromic acid in the same amount of water (12 g) and 60 g of glacial acetic acid is added dropwise with stirring over a period of 4 hours to a solution of 25 g of tropine in 500 cc of glacial acetic acid that has been warmed to 60-70Ą and is maintained at this temp during the addition. Heat the mixture for a short time on a steam bath until all the chromic acid has disappeared, cool and make strongly alkaline with NaOH. Extract with six 500 cc portions of ether and evaporate the ether in vacuo to get an oil that crystallizes readily. Purify by converting to the picrate or fractionally distill, collecting the fraction at 224-225Ą at 714 mm vacuo. The tropinones can be used in the above formula (or in a formula that you have found elsewhere) to be converted to cocaine. Remember to recrystallize the 2-carbomethoxytropinone before converting to methylecgonine.
- Joined
- Jan 15, 2023
- Messages
- 1,580
- Solutions
- 4
- Reaction score
- 1,099
- Points
- 113
- Deals
- 1
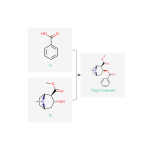
What Athena is trying to sell you is about 15 reactions in, 2corbomethoxy tropinone, it is only a benzoylation and an ethyl estefication away. It ain't cheap. It sounds like a hard Synthesis, but it's harder than it sounds. This Synthesis is not good. Your gonna spend a lot of money on the inactive enantiomer and the pseudotropane homolog. It needs to be done at -c° temperature. I doubt it could be done by a hobbyist. There are 3 better Synthesis on Google scholar at least
- Joined
- Jun 10, 2023
- Messages
- 7
- Reaction score
- 2
- Points
- 3
About Us
Our team brings together the best specialists from different fields.
We are ready to share our experience, discuss difficult issues and find new solutions.