You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Joined
- Dec 23, 2022
- Messages
- 320
- Solutions
- 1
- Reaction score
- 157
- Points
- 43
IPA is what I use. Warm it up and saturate the solution, freeze precipitate, filter the crystals, wash with sodium bisulfite to remove the last bit of benzaldehyde
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
is it necessary to freeze the crystals once they've precipitated? or do you freeze the solution they're dissolved in to get the precipitate? because i thought the slower the cooling process, the purer the crystals will be.
how should the crystals be washed with sodium bisulfite? should the crystals be dissolved in IPA first?
any help is appreciated.
how should the crystals be washed with sodium bisulfite? should the crystals be dissolved in IPA first?
any help is appreciated.
- Joined
- Aug 8, 2022
- Messages
- 675
- Reaction score
- 510
- Points
- 63
- Deals
- 5
Yes, freezing lower the solubility of the sol. thus extracting as much of your product as possible.it goes like this : from impure crystal -> dissolve in minimal amount of hot alcohol -> pop in the freezer .It's somewhat true, sol. of p2np in organic solvents need slow cooling to crystalize properly, in alcohol not so much and result is fine directly in the freezer.
I think he means washing the liquid with sodium bisulfate aq. sol. OR maybe washing of the crystals directly after filtering with the same sol.
I think he means washing the liquid with sodium bisulfate aq. sol. OR maybe washing of the crystals directly after filtering with the same sol.
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
- Language
- 🇬🇧
- Joined
- Jan 15, 2023
- Messages
- 577
- Reaction score
- 314
- Points
- 63
Careful, the washing must be done with saturated aqueous Na BISULFITE; bisulfate nor metabisulfite won't work even though sodium metabisulfite+H2O results in sodium bisulfite. With precise calculations one should be able to obtain the desired solution which is very useful in the purification of carbonyls because it forms an addition compound with most of them (benzaldehydes at least) which is solid and can be filtered out so for this very purpose is discarded but it can be done the other way around and then acidify or basify the addition compound to yield the carbonyl in purer form. Not having sodium bisulfite in reach I remember purifying 2MeObenzaldehyde (after synthesis from salicyladehyde with dimethylsulfate) with a solution meticulously prepared from sodium sulfite and a small and precise volume of GAA (idk the refference but surely enough it was an old book). The insufficient data I provided can be further researched by anyone or calculated by a more competent chemist than I am. Hope it spare's some frustration.
- Joined
- Dec 31, 2022
- Messages
- 128
- Reaction score
- 160
- Points
- 43
Procedure
Requirements
- Sodium bicarbonate (NaHCO3)
- Ethanol [EtOH /C2H6O/CH3CH2OH] / Methanol [MeOH /CH4O/CH3OH] / Isopropyl alcohol [IPA /C3H8O/CH3CHOHCH3 / (CH3)2CHOH]
- Activated carbon
Acid :
- Add a small P2NP sample to a flask
- Dissolve completely in EtOH/MeOH/IPA
- Prepare a solutionNaHCO3 ( 5-10%), pour it to the flask until pH = 8-9
- Stir 5-10 min
- Separate the organic layer
Activated carbon :
- Dissolve completely in EtOH/MeOH/IPA
- Stir 30-60 min, better yet hours
- Filter
- Freeze
- Filter
Benzaldehyde :
- Dissolve completely in EtOH/MeOH/IPA
- Prepare a solutionNaHCO3 ( 5-10%) and pour it to the flask
- Leave 5 min (observe precipitation)
- Filter
- Freeze
- Filter
Theory
References :
- Acetic acid [AcOH /C2H4O2 /CH3COOH]
- Sodium acetate [NaOAc /C2H3NaO2 /CH3COONa]
- Water (H2O)
- Carbon dioxide (CO2)
- Benzaldehyde [C7H6O/C6H5CHO]
- Benzylamine [C7H9N/C6H5CH2NH2]
- Sodium benzoate [C7H5NaO2 /C7H5O2Na/C6H5COONa/NaC6H5COO/NaC7H5O2]
NaHCO3 is alkaline. If an acid is present an acid-base reaction undergoes between the sodium Na+ cation and the acid anion, forming an insoluble acid salt andH2O. With AcOH its NaOAc.CO2 is released during the neutralization.
AcOH +NaHCO3 → NaOAc +H2O+CO2
C7H6Ois a common impurity in P2NP which arises from the oxidation ofC7H9N, that is commonly used in the synthesis of P2NP or simply the unreacted reagent from the most commonly used Knoevenagel condensation. To remove it a base-catalyzed condensation occurs.NaHCO3 acts as a base, deprotonatingC7H6Oforming its anionC7H6O-which then reacts with the sodium Na+ cation forming a benzoate salt -C7H6O,H2OandCO2.
C7H6O+NaHCO3 →C7H5NaO2 +H2O+CO2
As for the activated carbon its tiny pores adsorb various impurities and trap them on its surface. This can be applied during the purification of many compounds or elements. It has to be highly pure for this to work and to avoid further contamination of your sample.
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
thank you so much for this.
when doing the activated carbon stage, do you mean to use a layer of activated carbon in a buchner funnel sandwiched between 2 pieces of filter paper? if so, how thick should the layer of it be? 1cm?
when doing the activated carbon stage, do you mean to use a layer of activated carbon in a buchner funnel sandwiched between 2 pieces of filter paper? if so, how thick should the layer of it be? 1cm?
↑View previous replies…
- Joined
- Dec 31, 2022
- Messages
- 128
- Reaction score
- 160
- Points
- 43
Not really. You put activated carbon at the bottom of a flask and add the solution. The activated carbon comes in direct contact with the solution similar to using a drying agent. The only difference from a technical point of view is that activated carbon adsorbs whereas a drying agent absorbs. Adsorbtion means adhering to the surface rather than sucking up.
This is why the activated carbon should be as pure as possible. If you use a shitty cheap one it will only contaminate whatever you're trying to purify with dust. It's really cheap. Saving a few cents on it is just plain stupid in this case.
As for the amount to use it depends on how and of what you're purifying. It's really an educated guess where ideally you test samples that weight the same progressively adding more activated carbon. Then you extract the product, check for impurities and weight it. Ideally you test with a spectrometer but without it there are test to check the estimated purity. The simplest one is to visually check the integrity of the crystals and the color. Eventually you reach a point where the product loses weight. This is because the activated carbon adsorbed it too. You want to measure the exact amount which gives you the best purity but does not adsorb a noticeable amount of your wanted product.
This is why the activated carbon should be as pure as possible. If you use a shitty cheap one it will only contaminate whatever you're trying to purify with dust. It's really cheap. Saving a few cents on it is just plain stupid in this case.
As for the amount to use it depends on how and of what you're purifying. It's really an educated guess where ideally you test samples that weight the same progressively adding more activated carbon. Then you extract the product, check for impurities and weight it. Ideally you test with a spectrometer but without it there are test to check the estimated purity. The simplest one is to visually check the integrity of the crystals and the color. Eventually you reach a point where the product loses weight. This is because the activated carbon adsorbed it too. You want to measure the exact amount which gives you the best purity but does not adsorb a noticeable amount of your wanted product.
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
Is the activated carbon introduced to the solution at step 2 and stirred for several hours?
when filtering do i simply use a piece of filter paper and a buchner funnel? (i presume this is to get rid of any remaining activated carbon.)
how is the filtration done the second time around, given that the p2np has at this point already crystalized?
when filtering do i simply use a piece of filter paper and a buchner funnel? (i presume this is to get rid of any remaining activated carbon.)
how is the filtration done the second time around, given that the p2np has at this point already crystalized?
- Joined
- Dec 31, 2022
- Messages
- 128
- Reaction score
- 160
- Points
- 43
I always use activated carbon at the very end.
As for the filtration you are correct to think that it is done to separate the activated carbon from the solution. A Büchner funnel and a filter paper does the job just fine.
Taken into consideration the order of each step you remove the benzaldehyde using sodium bicarbonate which also neutralizes the acid. If there is acid you aim for ph 8-9, if not you add sufficient sodium bicarbonate until no more precipitation occurs. At this point you can filter whilst the P2NP stays dissolved in solution and use the activated carbon. Then you filter again and crystalize the P2NP. This way you perform the entire purification using the least IPA possible reusing the solvent throughout each step.
I've just written each step on it's own in case someone would want to do only one of them.
As for the filtration you are correct to think that it is done to separate the activated carbon from the solution. A Büchner funnel and a filter paper does the job just fine.
Taken into consideration the order of each step you remove the benzaldehyde using sodium bicarbonate which also neutralizes the acid. If there is acid you aim for ph 8-9, if not you add sufficient sodium bicarbonate until no more precipitation occurs. At this point you can filter whilst the P2NP stays dissolved in solution and use the activated carbon. Then you filter again and crystalize the P2NP. This way you perform the entire purification using the least IPA possible reusing the solvent throughout each step.
I've just written each step on it's own in case someone would want to do only one of them.
- Joined
- Mar 9, 2023
- Messages
- 111
- Reaction score
- 44
- Points
- 28
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
i think i made a mistake when doing the activated carbon step - without knowing how much to add, i put in slightly more than an amount of the activated carbon that was equal to the amount of P2NP. the solution was very thick and after i tried to filter it with the buchner funnel nothing would happen. it is just a black sludge and i can't seperate the activated carbon from the P2NP. can this be salvaged?
- Joined
- Aug 8, 2022
- Messages
- 675
- Reaction score
- 510
- Points
- 63
- Deals
- 5
ofcourse, heat it add more solvent optionally and filter hot
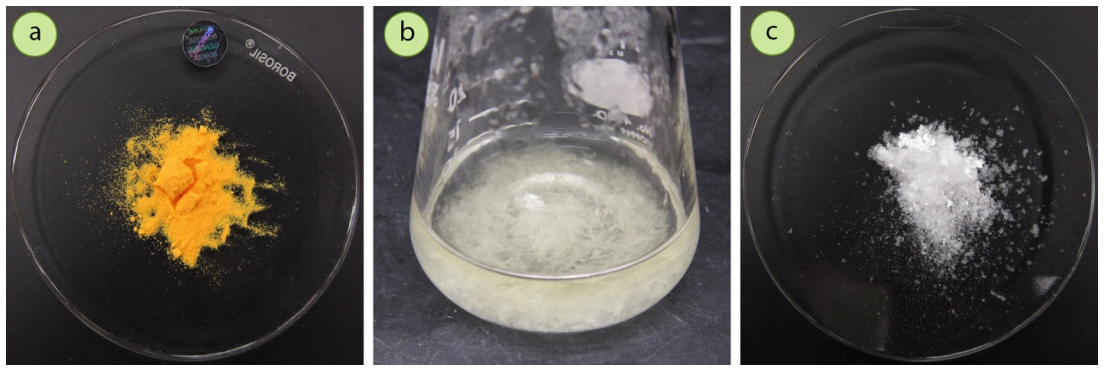
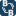 bbgate.com
bbgate.com
Recrystallization and hot filtration
Recrystallization. In chemistry, recrystallization is a technique used to purify chemicals. By dissolving both impurities and a compound in an appropriate solvent, either the desired compound or impurities can be removed from the solution, leaving the other behind. It is named for the crystals...
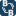 bbgate.com
bbgate.com
↑View previous replies…
- Joined
- Aug 8, 2022
- Messages
- 675
- Reaction score
- 510
- Points
- 63
- Deals
- 5
Try gravity filtration on filter paper maybe too instead of vacuum, you could also use a flocculant I guess but try the simple stuff first
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
i tried it again with more solvent, and i have a layer of what looks to be charcoal on the filter paper, and i have a black colored solution. but if the p2np was in the black solution then shouldn't it have crystalized when it reached room temp? because it didn't. i've put it in the freezer to see if anything will precipitate out.
- Joined
- Aug 8, 2022
- Messages
- 675
- Reaction score
- 510
- Points
- 63
- Deals
- 5
Not if it’s a lot of solvent, it won’t crystalize at rt in matter of hours
- Language
- 🇬🇧
- Joined
- Aug 19, 2022
- Messages
- 264
- Reaction score
- 27
- Points
- 28
About Us
Our team brings together the best specialists from different fields.
We are ready to share our experience, discuss difficult issues and find new solutions.
